Cordguard™ Umbilical Cord Management
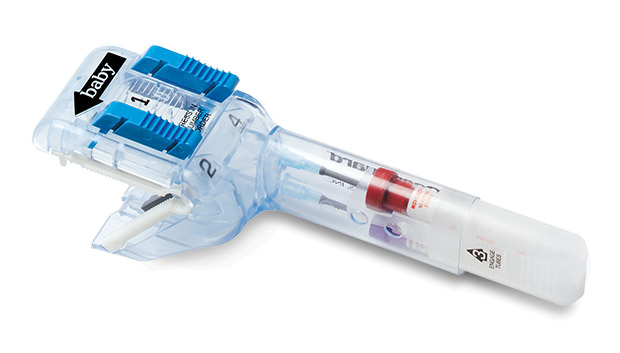
Cordguard™ is an innovative all-in-one unified system designed for umbilical cord clamping, cutting and blood sampling/collection. It provides an excellent neonatal blood sample while minimizing clinician exposure to blood, and a secured cord can help deliver the placenta.
- Quick and easy 4-step activation.
- Enclosed collection reservoir for obtaining clean, uncontaminated umbilical cord blood.
- Fully enclosed sharps.
- Reliable, secure cord clamp.
- Ergonomic handle assists in delivery of placenta.
- Protective sheath keeps tubes clean for labeling.
- Two vacuum tubes conveniently slide from handle when full.
CLINICAL NEED
"There are a number of instances where the collection of blood from the neonate is difficult or impossible, thus placing strong emphasis on the collection of umbilical cord blood from every delivery." Source: The Collection of Umbilical Cord Blood, Communicore, 1992
CLINICAL USE
"Cord blood is the best medium for general purpose diagnostic tests indicative of status in the neonate."
Tests are commonly performed for:
— Routine blood work (i.e. Type, pH, CBC, Direct coombs)
— Infectious diseases
— Abnormalities/fetal status
— Atopic allergies
— Detection of heavy metals (i.e. Lead)
— Genetic analysis
Potential applications of cord blood are broader in scope and include:
— Generation of red blood cells
— Gene therapy
"Determination of pH and blood gas values from umbilical arterial and venous blood provides an objective method of assessing fetal acid-based status at the time of birth and is a useful adjunct to the Apgar score in assessing the immediate condition of the newborn.
...umbilical venous blood can be utilized. In a truly asphyxiated fetus, the pH of both arterial and venous blood will most likely be very low." Source: ACOG Technical Bulletin #127
EXPOSURE ISSUES
It's estimated that more than 6.2 million Americans are infected with HIV, Hepatitis B or Hepatitis C. Source: Centers for Disease Control and Prevention Publication, Vol. 23, No. 3, Sept. 1994
Exposure Risks:
96% of clinician respondents have been exposed to umbilical cord blood.
84% of respondents are concerned about exposure to umbilical cord blood.
39% of vaginal deliveries expose at least one healthcare worker.
50% of cesarean deliveries expose at least one healthcare worker.
Source: UTMD Market Research, 1994; American Journal of Ob/Gyn 1992, 167:
703-8
"All procedures involving blood or other potentially infectious materials shall be performed in such a manner as to minimize splashing, spraying, spattering and generation of droplets of these substances." Source: OSHA Regulations on Bloodborne Pathogens, #1910. 1030, Federal Register, Vol. 56, No. 235.
Cordguard™ Umbilical Cord Management Systems
| Model Number |
Description |
Unit of Measure |
Image |
| CRD-201 |
Cordguard™ Umbilical Cord Clamping, Severing, and Blood Collection System. |
10/BOX |
 |
For more information, please contact us by EMAIL or call 1-800-533-4984 | 1-801-566-1200.

